Department Environmental Chemistry
Isotope fractionation associated with direct and sensitized photolysis of chloroanilines
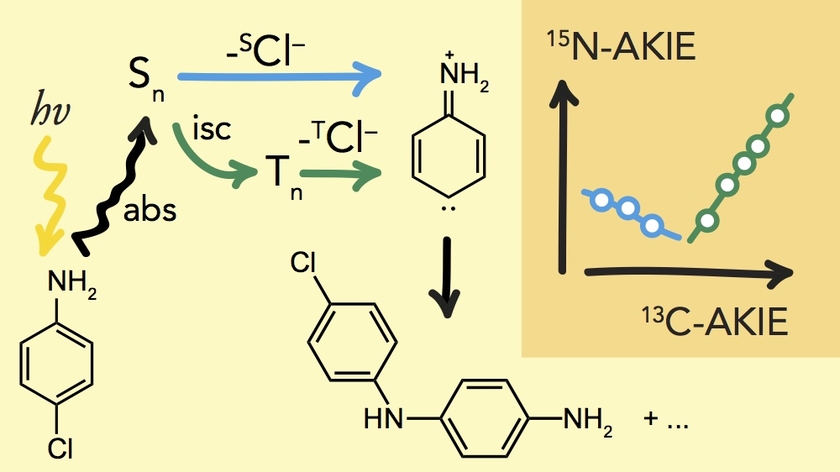
Isotope fractionation associated with the photochemical transformation of organic contaminants is not well understood and can arise not only from bond cleavage reactions but also from photophysical processes.
We are investigating the photolytic dechlorination of 2-Cl-, 3-Cl-, and 4-Cl-aniline as model compound for organic micropollutants that degrade in photochemical processes.
The reaction of each chloroaniline isomer is accompanied by distinctly different and highly variable C and N isotope fractionation due to spin selective isotope effects. The isotope fractionation trends associated with photolytic dechlorination strongly contrasts dechlorination in thermal reactions and offers new opportunities for fingerprinting photochemical processes in contaminated surface waters.