Department Environmental Chemistry
Pharmaceutical Fate
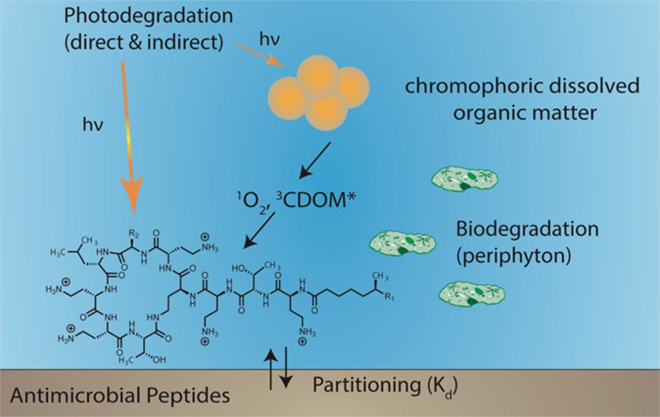
To investigate the fate processes of pharmaceuticals in surface waters we study transformation pathways and kinetics by abiotic and biotic processes. We quantify direct phototransformation kinetics as well as indirect photochemical processes involving reactive intermediates to derive second-order rate constants that are system-independent. We also determine the biological degradability of organic pollutants for examples with freshwater biofilm communities. We are particularly interested in the contribution of extracellular enyzmes on transformation processes of compounds that are note readily taken up by cells. These compounds include antimicrobial peptides and natural toxins.
Recent projects:
We recently transferred our knowledge gained for small molecule photochemistry to macromolecular pollutants (> 500 Da), which present an added challenge due to their structurally complexity. In a recent study we focused on antimicrobial peptides (AMPs). AMPs are increasingly important as a last resort against multi-drug resistant bacteria due to resistance formation towards conventional antibiotics. While AMPs have been administered as antibiotics and growth promotors in feedstock since the 1960s and were reconsidered for human medicine by the EMA in 2013, details about their mobility and persistence in the environment remain unknown. Our findings of sorption behaviour, photo- and biotransformation suggest that these processes play a critical role in the fate of the AMPs bacitracins, daptomycin, and polymyxins in environmental systems.
For more information take a look at the following publication.
We previously investigated the kinetic solvent isotope effect (KSIE) as it is typically utilized in environmental photochemistry to elucidate whether a compound is susceptible to photooxidation by singlet oxygen (1O2). This KSIE manifests itself in the known difference in 1O2 lifetime in water (H2O) versus heavy water (D2O). We demonstrate an additional enhancement in D2O beyond reaction with 1O2 contributed significantly to the observed KSIE for selected pharmaceuticals. The enhancement was ascribed to slower reduction of transient radical species due to H/D exchange at DOM’s phenolic antioxidant moieties. Other pollutants with quenchable radical intermediates may also be susceptible to such an additional KSIE, which has to be considered when using the KSIE as a diagnostic tool. For more information take a look at the following publicationhttps://www.eawag.ch/typo3/#_msocom_1.
Another project focused on electron transfer properties between compounds of interest and dissolved organic matter (DOM). We demonstrated that the dual roles of DOM, acting as oxidant and antioxidant, significantly influences the photochemical fate of non-steroidal anti-inflammatory drugs and the amino acid tryptophan. We used steady-state as well as laser-flash photolysis and high-resolution mass spectrometry to mechanistically elucidate reaction pathways in these studies. For more information take a look at the following publicationhttps://www.eawag.ch/typo3/#_msocom_1.
Publications
Additional publication
Elisabeth M.-L. Janssen, Emily Marron, Kristopher McNeill (2015) Aquatic photochemical kinetics of benzotriazole and structurally related compounds. Environmental Science: Processes and Impacts, 17, pp. 939-946. DOI: 10.1039/C5EM00045A
