Department Environmental Chemistry
Understanding of the unusual redox buffer behaviour of clay minerals
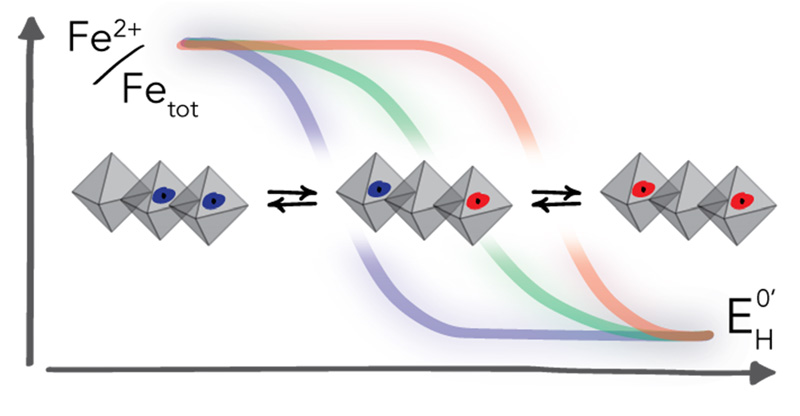
Electron transfer reactions involving Fe(II)/Fe(III) couples associated with iron-bearing clay minerals profoundly impact the environment by influencing organic carbon cycling, microbial activity, rock weathering and diagenesis, and corrosion. These redox reactions are also particularly important for groundwater remediation efforts because they produce Fe(II) that can abiotically reduce several classes of oxidized environmental contaminants to less toxic or less mobile forms, including radionuclides, toxic metals and metalloids, and various organic pollutants.
Rates and extents of these redox reactions strongly depend on the speciation and coordination of Fe(II)/Fe(III), which has important consequences for interpreting the roles that iron plays in biogeochemical cycles as well as pollutant dynamics. In contrast to iron minerals such as iron (oxyhydr)oxides, changes of Fe oxidation state in clay minerals are not necessarily coupled to reductive dissolution. Instead, electron transfer to and from Fe primarily leads to structural re-arrangements of the mineral structure.
In our research, we evaluate whether this feature is responsible for observations of the unusually large range of reduction potentials over which Fe in clay minerals can be involved in redox reactions. Our work combines the evaluation of Fe binding and redox state by spectroscopic and microscopic analyses with the electrochemical characterization of Fe redox reaction over the reduction potential range found in subsurface environments. In addition to studying reference clay minerals, our recent work extents to synthetic materials with well-defined iron coordination.
