Department Water Resources and Drinking Water
Tritium (3H)
Tritium is a very important environmental tracer for the study of lakes, oceans and groundwater.
The tritium (3H) isotope
τ½
Tritium (3H) is the radioactive isotope of H that decays by β - decay to 3He (half-life):
= 4500 days = 12.32 years (Lucas and Unterweger, 2000):
3H → 3He λ = ln(2)/τ½
with the decay constant:
= 0.05626 yr-1 = 1.7828 10-9 s-1
For modern water isolated from the atmosphere, 3H is a major source of 3He. For each 3H that decays one 3He atom is produced. The natural abundance of 3H is usually expressed in Tritium Units (TU):
1 TU = 1 3H atoms per 1018 H atoms = 1.100 10-19 mol/gpure water = 66'846 atoms/gpure water
Sometimes 3H concentrations are also expressed in activities (IAEA, 1992):
1 Bq/L = 8.47 TU; 1 pCi/L = 0.313 TU
The conversion from TU into cm3STP/g is given by:
1 cm3STP / gwater = 4.0193 1014 TU / (1-S/100)
where S is the salinity of the water (in ‰).
Since tritium is essential for the 3H/3He dating method, its properties will be discussed here in some detail.
Tritium in the environment
Tritium is probably the single most important environmental isotope for the study of lakes, oceans and groundwater. It is produced naturally by the interaction of cosmic rays with nitrogen and oxygen, mainly in the upper atmosphere, and, after oxidation to 1H3HO, takes part in the hydrological cycle. In the atmosphere the average production rate of natural 3H is about 2500 3H atoms m-3 s-1 (Craig and Lal, 1961; Rozanski et al., 1991), but varies with the geomagnetic latitude. Before significant amounts of 3H were injected into the atmosphere through man's nuclear activities, precipitation had a natural background of around 5 TU (Kaufman and Libby, 1954; Craig and Lal, 1961; Lal and Peters, 1962; Roether, 1967). During the early 1960's, when the effects of atmospheric testing of nuclear weapons peaked, 3H concentrations of over 2000 TU were measured. Since most of the nuclear weapons tests were performed in the northern hemisphere, even today the 3H distribution is strongly asymmetric between the northern and southern hemisphere (Figure 1). Today atmospheric background levels in the northern hemisphere are between about 5 and 30 TU, and in the southern hemisphere between 2 -10 TU (IAEA/WMO, 2006).
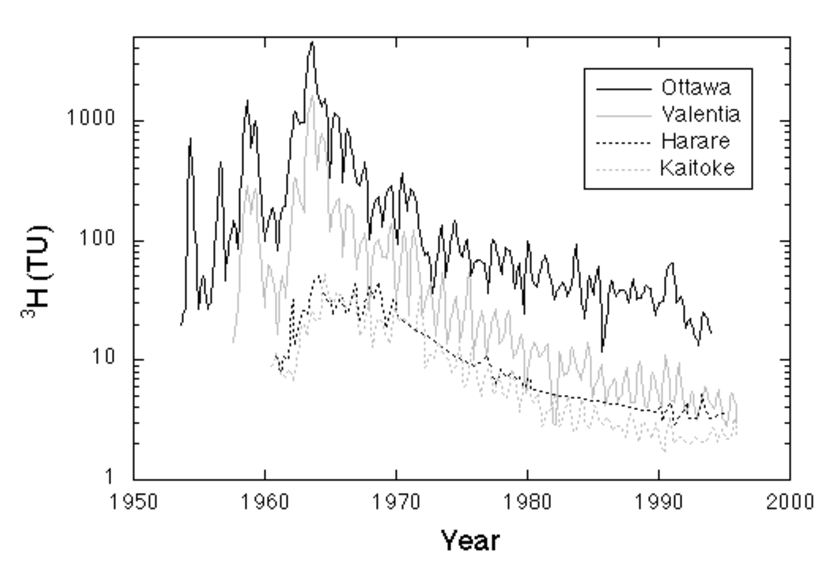
In groundwater 3H is a good indicator of the presence of or contamination by recently infiltrated water (younger than 50 years). Subsurface production of 3H occurs mainly by the reaction 6Li (n, a) 3H in the rock matrix and depends therefore largely on the Li content and on the neutron flux. The produced 3H atom migrates from the site of origin and finally exchanges a H atom in a water molecule (Andrews et al., 1989). Since the produced 3H decays to 3He, the subsurface produced 3H concentration in groundwater represents an approximate balance between production and decay. For example, Andrews et al. (1989) calculated a maximum 3H concentration from in situ production of 0.7 TU in the Stripa groundwaters.
Measuring tritium
Tritium concentrations in waters can be determined using low-level liquid-scintillation counting techniques. The lower 3H levels in recent precipitation demand more precise analytical methods than in the 1970-1990 period. Modern analytic techniques, such as prior isotopic enrichment (Weiss et al., 1976) or the determination of 3H by 3He ingrowth (Clarke et al., 1976), allow 3H detection as low as 0.1 to 0.005 TU (Bayer et al., 1989). Limitations in the use of 3H alone, as a water mass tracer, arise from its radioactive nature and from dispersion of the bomb signal in the system investigated, leading to difficulties in interpreting the 3H distributions as a measure of the residence time. Therefore 3He is additionally measured to obtain water ages (see 3H/3He water ages).
References
- Lucas, L. L. and M. P. Unterweger (2000) Comprehensive review and critical evaluation of the half-life of Tritium. J. Res. Natl. Inst. Stand. Technol. 105(4): 541-549.
- IAEA. (1992) Statistical treatment of data on environmental isotopes in precipitation. International Atomic Energy Agency, IAEA.
- Craig, H. and D. Lal (1961) The production rate of natural tritium. Tellus, 13(1), 85-105.
- Rozanski, K., R. Gonfiantini and L. Araguas-Araguas (1991) Tritium in the global atmosphere: Distribution patterns and recent trends. J. Phys. G: Nucl. Part. Phys., 17, S523-S536.
- Kaufman, S. and W. F. Libby (1954) The natural distribution of tritium. Phys. Rev., 93(6), 1337-1344.
- Lal, D. and B. Peters (1962) Cosmic ray produced isotopes and their application to problems in geophysics. Elementary Particle and Cosmic Ray Physics, 6, 1-74.
- Roether, W. (1967) Estimating the tritium input to groundwater from wine samples: Groundwater and direct run-off contribution to Central European surface waters. In: Isotopes in Hydrology (Ed. by IAEA), pp. 73-79. IAEA, Vienna.
- IAEA/WMO. (2006) Web Site of the Global Network of Isotopes in Precipitation (GNIP) and Isotope Hydrology Information System (ISOHIS). http://isohis.iaea.org/, IAEA, Vienna.
- Andrews, J. N., S. N. Davis, J. Fabryka-Martin, J.-C. Fontes, B. E. Lehmann, H. H. Loosli, J.-L. Michelot, H. Moser, B. Smith and M. Wolf (1989) The in situ production of radioisotopes in rock matrices with particular reference to the Stripa granite. Geochim. Cosmochim. Acta, 53, 1803-1815.
- Weiss, R., W. Roether and G. Bader (1976) Determination of blanks in low-level tritium measurement. International Journal of Applied Radiation Isotopes, 27, 217-225.
- Clarke, W. B., W. J. Jenkins and Z. Top (1976) Determination of tritium by mass spectrometric measurement of 3He. Int. J. appl. Radiat. Isotopes, 27, 515-522.
- Bayer, R., P. Schlosser, G. Bönisch, H. Rupp, F. Zaucker and G. Zimmek (1989) Performance and Blank Components of a Mass Spectrometric System for Routine Measurement of Helium Isotopes and Tritium by the 3He Ingrowth Method. Springer-Verlag.
