Archive detail
Virtual fish instead of animal testing
July 18, 2022 |
The new national research programme “Advancing 3R – Animals, Research and Society”(NRP 79) of the Swiss National Science Foundation promotes scientific projects that contribute to improving, reducing or replacing animal experiments in Swiss research. At the beginning of June, 23 of the submitted projects were selected to receive a total of CHF 14.9 million in funding. Among them is a joint project of the aquatic research institute Eawag and the University of Utrecht. The project, which is being funded with almost CHF 1 million, is scheduled to run for four years and has the objective of replacing animal testing involving fish for the approval of chemicals in the future with a combination of in-vitro tests and computer models. The project is coordinated by Kristin Schirmer, Head of the Environmental Toxicology department of Eawag and titular professor at ETH Zurich and EPFL, and Bernhard Truffer, until recently Head of the Environmental Social Sciences department of Eawag and Professor at the University of Utrecht.
Eawag test with fish cells replaces animal experiments
Under the leadership of Kristin Schirmer, Eawag has been working for many years to replace animal experiments on fish with in-vitro methods. These are toxicity tests based on fish cells grown in the laboratory. These cells can be used to study how toxic certain chemicals are to fish. This is an important result, which determines, for example, whether new substances can be approved for the market. A test developed by Schirmer’s team based on rainbow trout gill cells was released last year by the Organisation for Economic Cooperation and Development (OECD) as the latest guideline in the field of environmental toxicology. "We chose gill cells because it is the gills that first come into contact with chemicals in water due to their large surface area in the fish,” explains Schirmer. "So by observing how the gill cells are damaged by a chemical, we can predict how that chemical would affect a living fish."
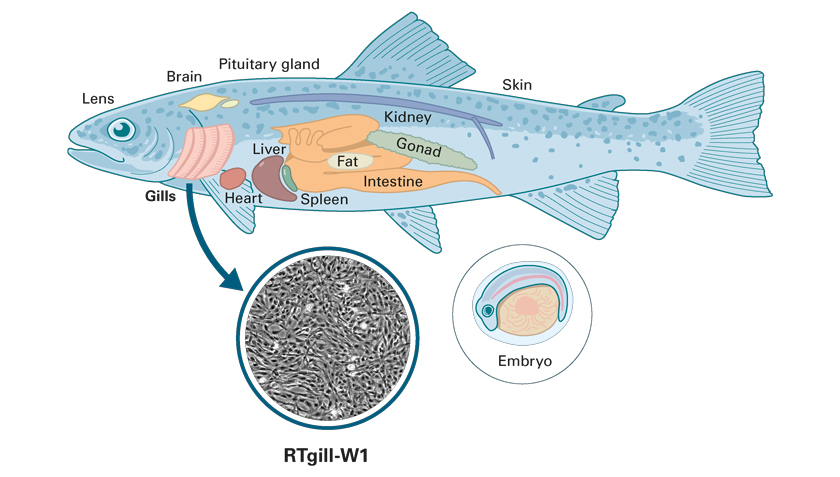
A test developed at Eawag based on rainbow trout gill cells (RTgill-W1) was released last year by the OECD as the latest guideline in the field of environmental toxicology. However, a more comprehensive picture of how chemicals can affect the fish can only be obtained by considering other important organs in addition to the gills. Cell lines already exist for all the organs shown. Some of them are to be further developed as part of the NRP project. (Graphics: zeichenfabrik)
The virtual fish makes a comprehensive view possible
However, an even more comprehensive picture of how chemicals can affect the fish can only be obtained by considering other important organs in addition to the gills. The researchers are now pursuing this objective as part of NFP 79. They are working to this end, for example, on tests based on the intestine or nerve cells of rainbow trout. A look at the molecular level, i.e. at proteins or RNA molecules, also helps to complete the picture.
Ksenia Groh, who is involved in the project as a research group leader, explains: “Our vision is that the data from the in-vitro tests on cells of all major organs will flow into a single computer model, the virtual fish.” This should enable detailed and well-founded statements to be made on how chemicals affect fish – without having to resort to experiments on living fish.
Cooperation with partners in practice
An important part of the project is the involvement of the chemical industry as well as the authorities responsible for the approval of new chemicals. “We want to develop the virtual fish together with them in order to align the process from development to application at the regulatory level to the effective needs – which in the case of the gill test took twelve years –and thus speed it up,” says Groh. Bernhard Truffer, an expert in the field of technical innovations, provides the know-how needed to shape the cooperation and exchange with the practical partners. He notes: “Early involvement of the players is important so that the requirements posed by later implementation can already be taken into account during development of the methods. In this way, animal testing can be replaced more quickly in practice.” The team is supported by Jarno Hoekman, also a professor of innovation at the University of Utrecht.
Colette vom Berg, who is involved in the project as a research group leader, summarises:
“For some time now, Eawag has been doing research with many partners to develop tests that make experiments on living fish unnecessary. With this great project, we have the opportunity to combine different approaches and close gaps.”
One postdoctoral and two PhD positions are currently advertised as part of the project.
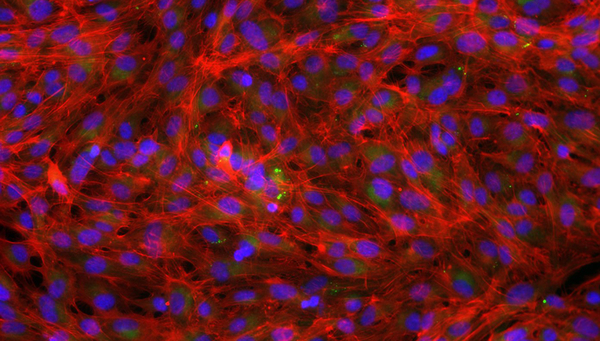
Cover picture: Rainbow trout gill cells were fixed and stained using molecular probes for nuclei (blue), lipid (green) and actin (red). These cells were healthy control cells unexposed to chemical stimuli. (Photo: Vivian Lu Tan, Eawag)