Department Process Engineering
Electrochemical Ammonia Removal
Most of the nitrogen removal processes in wastewater treatment are biological. While biological processes are energy efficient and do not require large amounts of chemical additives, they are not well suited for irregular inflow conditions as they might occur in very small decentralized reactors. Especially long phases without any inflow can lead to a complete process breakdown.
An alternative to biological nitrogen removal is electrolysis. Electrochemical ammonia oxidation was investigated more than 30 years ago, but the best available electrodes were made of platinum, which is expensive and is deactivated very quickly. For some years now, more resistant electrodes − dimensionally stable anodes (DSA) and boron-doped diamond electrodes (BDD) − have been available, which render electrolysis an attractive alternative in small reactors:
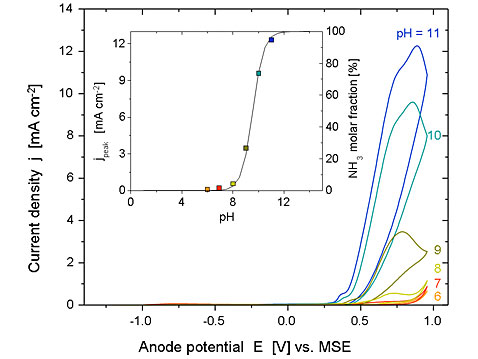
Cyclic voltammograms recorded on Ni/Ni(OH)2 in the presence of ammonia at various pH values (scan rate 100 mV s-1; 150 mM NH4ClO4 + 1M NaClO4 + NaOH, 25°C). The inset shows: (■) the current peak density as a function of pH and (―) the mole fraction of the ammonia, as a function of pH.
Electrochemical treatment can combine the degradation of organic substances, ammonia and the deactivation of pathogens. However, the process pathways are often complex and not all electrode materials are suitable for the degradation of ammonia. In laboratory experiments, we tested DSA (IrO2 film, and Ti/PtOx-IrO2), BDD and nickel electrodes. So far, it is not clear, whether DSA or BDD are better suited for urine treatment. Nickel electrodes are not suitable, because they corrode and release nickel ions into the solution.
In ammonium solutions without chloride, the electrolysis rate decreased with the pH value of the solution, indicating that ammonia (NH3) was the actual reactant for all three electrodes. In ammonia solutions with chloride, ammonium degradation also occurred at low pH values due to the production of hypochlorous acid and the subsequent chlorination of ammonium (breakpoint chlorination). In contrast to conventional breakpoint chlorination, much less chloramines were produced on Ti/PtOx-IrO2, because the reaction took place close to the electrode surface, where the hypochlorous acid concentrations are so high that the chloramines are oxidized very quickly to molecular Nitrogen.
Literature
Kapałka A., Katsaounis A., Michels N.-L., Leonidova A., Souentie S., Comninellis C., Udert K.M. (2010) Ammonia oxidation to nitrogen mediated by electrogenerated active chlorine on Ti/PtOx-IrO2. Electrochemistry Communications 12(9), 1203-1205..
Kapałka A., Cally A., Neodo S., Comninellis C., Wächter M., Udert K.M. (2010) Electrochemical behavior of ammonia at Ni/Ni(OH)2 electrode. Electrochemistry Communications 12(1), 18-21.
Kapałka A., Fierro S., Frontistis Z., Katsaounis A., Frey O., Koudelka M., Comninellis C., Udert K.M. (2009) Electrochemical behaviour of ammonia (NH4+/NH3) on electrochemically grown anodic iridium oxide film (AIROF) electrode. Electrochemistry Communications 10(8), 1590-1592.
