Department Environmental Chemistry
Extracellular enzymes
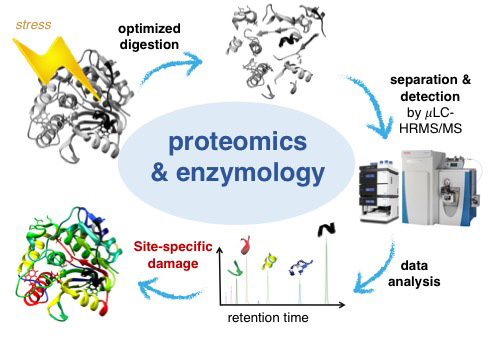
Extracellular enzymes are major drivers of biogeochemical nutrient and carbon cycling in surface water. We investigate the activity of these enzymes to degrade pollutants (projects on pharmaceutical fate and natural toxins) study the decay of the enzymes themselves. Photoinactivation and biotransformation are the major inactivation process of these enzymes. We study the underlying molecular changes that cause inactivation of enzymes. We track changes in activity during exposure to external stress (i.e., light and oxidants) and combine this with information on molecular modifications of the enzymes‘ structure.
We demonstrated how light exposure leads to a rapid loss of phosphatase, aminopeptidase and glucosidase activities of biofilm samples and model enzymes. An optimized proteomics approach allowed simultaneous observation of inactivation and molecular changes. Site-specific fingerprints of degradation kinetics have been generated and visualized in the three-dimensional proteins and intramolecular reactions could be traced within the molecule.
We included comprehensive suspect screening of oxidation products from discrete reactions of enzymes with photochemically derived singlet oxygen in surface waters.
Currently, we collaborate with colleagues to study site-specific damage of oxygenases that are involved in hydroxylating persistent (poly)cyclic chlorinated and nitrated hydrocarbons with the group of Thomas Hofstetter (Project on Enzyme Mechanisms and Kinetics of Organic Contaminant Oxygenation).
Publications
Additional publication
Elisabeth M.-L. Janssen and Kristopher McNeill (2015) Environmental photooxidation of extracellular phosphatase and the effects of dissolved organic matter. Environmental Science and Technology, 49 (2), pp. 889-896. DOI: 10.1021/es504211x.
Rachel A. Lundeen, Elisabeth M.-L. Janssen, Chiheng Chu, Kristopher McNeill (2014) Environmental photochemistry of amino acids, peptides and proteins. Chimia, 68 (11), pp. 814-817. https://doi.org/10.2533/chimia.2014.812.
Elisabeth M.-L. Janssen, Paul R. Erickson, Kristopher McNeill (2014) Dual roles of dissolved organic matter as sensitizer and quencher in the photooxidation of tryptophan. Environ. Sci. Technol., 48(9), pp. 4916-24. DOI:10.1021/es500535a
